Power Plate® My5™
When you want a proven, low-stress exercise and rehabilitation solution at home, the sleek and elegant Power Plate® My5™ delivers effectively.
$8,495.00
In stock (can be backordered)
Fast forward to the body you want
Reach your goals and exceed your expectations
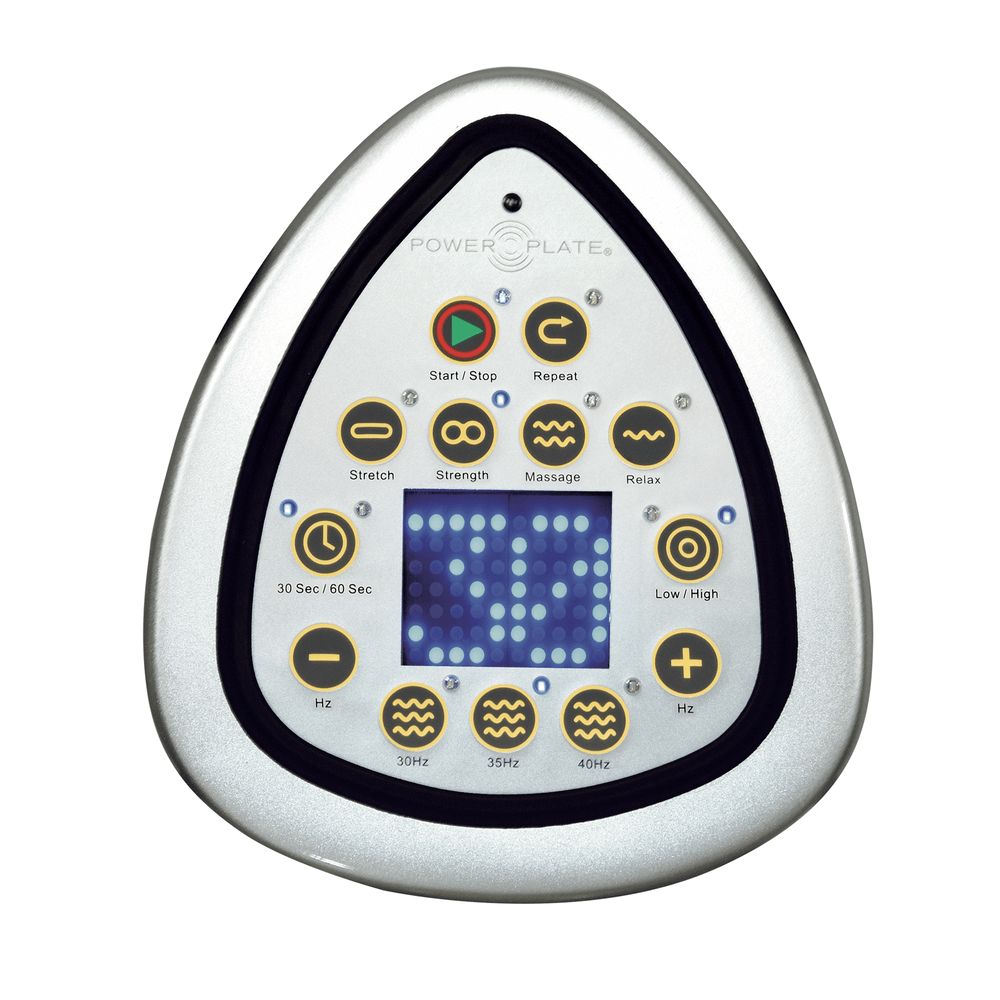
The Power Plate® My5™ model brings great full-body results to your home workouts in as little as 20 or 30 minutes a day, three days a week. With a frequency range of between 30-40HZ, it’s more customizable than the Power Plate® My3™ and it offers a larger plate surface, providing more exercise versatility. It also allows for a higher maximum load, has pre-programmed quick start buttons and a remote control.
The sleek design of the my5 combined with the technology creates a wonderful blend of science and elegance at home, making it easy for anyone at any fitness level to access better health.
FEATURES
- Grade: Consumer
- Time Selections: 30 / 60 seconds
- Frequency / Pre-set Frequencies: 30-40Hz / 35, 40, and 40 Hz
- Vibration Energy Output (amplitude): Low / High
- Operation: User-friendly interactive display
- PrecisionWave™ Technology: High-fidelity harmonic vibration system that provides uncompromising performance for unsurpassed results
- Dual Sync™ Twin Motor System: DualSync Twin Motor System maintains precise balance at any frequency and amplitude level, allowing perfect synchronization of vibration for maximum muscle response and efficiency
- Secondary timer and controls
- Quick start programs
What’s Included
- Hand Straps (set of 2)
- Rubber Mat Set (set of 3)
- Remote Control
- Instructions for Use
- Exercise Poster
- Dust Covers (column and platform)
- Power Cord
SPECIFICATIONS
- Dimensions (WxDxH): 27in x 36in x 58in / 68cm x 92cm x 148cm
- Platform Dimensions (WxD): 27in x 28in / 68cm x 72cm
- Weight: 151lb / 68kg
- Maximum Load: 300lb / 136kg
- Power Supply: 240 VAC, 50/60Hz, Universal Voltage
- Nominal Power: 160-320VA
Certifications:
- CE
- FDA listed as Class 1 device
- 510k exempt